Clinical Trial Regulation
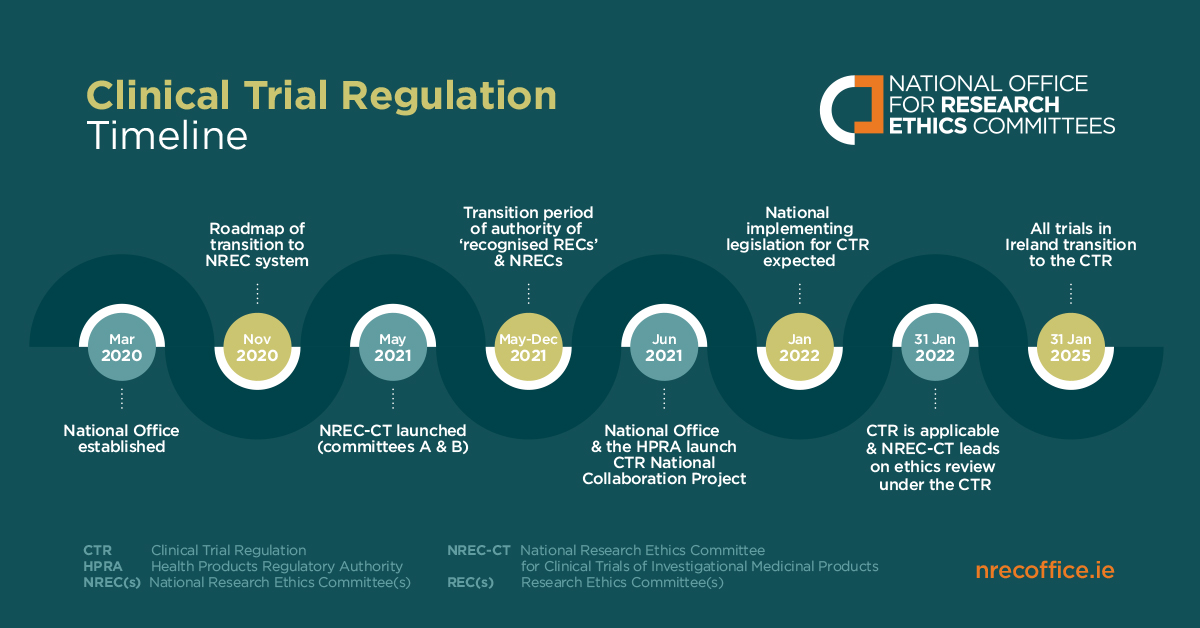
On 31 January 2022, the new Clinical Trial Regulation (CTR) entered into force. The new regulation requires that all clinical trial applications and assessments be managed through a new, unified online portal known as the Clinical Trial Information System (CTIS). The CTIS acts as a one-stop, centralised repository for all information relating to clinical trials in the EU.
Important to note: due to the new regulation entering into force, applications for clinical trials that come under the regulation can no longer be submitted directly to the National Research Ethics Committee for Clinical Trials (NREC-CT) or to the Health Products Regulatory Authority (HPRA). Instead, they must be submitted using the CTIS. Documentation requirements will also change under the CTR. Please see further information on documentation under Part II National Requirements here.
Ethics assessment of all Clinical Trials of Investigational Medicinal Products
On the back of the CTR entering into force:
- NREC-CT has taken on full responsibility of the ethics assessment of all new clinical trial applications.
- Local Research Ethics Committees retain reporting responsibility for ongoing trials that have not yet transitioned to the CTR or to the NREC system.
More information
Detailed information on part II requirements specified by the NREC-CT is available on our Part II National Requirements page.